
 |
Use the calculated data to fill in the table below.
|
Use these results to state whether -NO2 is an ortho-para directing substituent or a meta-directing substituent for electrophilic aromatic substitution. Explain whether or not this result agrees with what you learned in organic chemistry. Use any piece of structure drawing software to draw appropriate resonance structures.
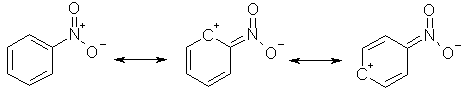 |
Since NO2 is an electron withdrawing group, a glance at the resonance structures shows that the positive charge becomes concentrated at the ortho-para positions. Thus these positions are deactivated towards electrophilic aromatic substitution. Hence, NO2 is a meta-director, as we all learned in organic chemistry.
Similarly, determine whether -CH3 is ortho-para directing or meta-directing substituent for electrophilic aromatic substitution. Explain whether or not this result agrees with what you learned in organic chemistry.
|
 |
The CH3 group donates electrons to the aromatic ring due to hyperconjugation. This excess charge becomes concentrated at the ortho-para positions, as shown by the resonance structures above. Thus, these positions become activated towards electrophilic aromatic substitution, and the CH3 group is an ortho-para director, as we learned in organic chemistry.