 |
In this exercise, you will search for an anomeric effect in 2-chlorotetrahydropyran.
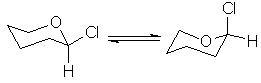 |
A. Use the WebMO editor to build 2-chlorotetrahydropyran, with the chlorine atom in the axial position. Use PM3 to optimize the geometry of the resulting conformation, and measure its final energy. Using the optimized geometry, construct the equatorial conformation by simply changing two dihedral angles by 180°. Use PM3 to measure the final energy of the second conformation, and report the heats of formation of each conformation in the table below.
B. Repeat the calculations for chlorocyclohexane.
C. Calculate DfGeq-DfGax for each compound and compare your results in a table with the experimental results.
|
For which compound does an anomeric effect exist? By comparing the structural differences between the two compounds, what factors likely cause the anomeric effect?