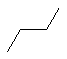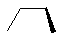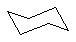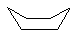Conformational Energy
Objective
The objective of this exercise is to calculate the strain energy incurred as a molecule
moves between various conformations.
Procedure
Use PM3, semi-empirical, calculations to calculate the strain energy of the following molecules.
The strain energy can be calculated by subtracting the heat of formation of the more stable species
from that of the less stable species. The strain energy should be a positive quantity.
|
| Molecule |
Strain Energy (kJ/mol) |
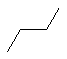 |
0 |
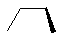 |
2.17 |
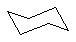 |
0 |
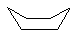 |
17.1 |
|
Explain whether of not your results agree with your "chemical intuition."
|
As we would expect, trans-butane is slightly more stable than its gauche counterpart. This is due
to the steric interactions between methyl group hydrogens the latter. Likewise, chair-cyclohexane
is significantly less strained than it boat counterpart, due again to the steric interactions of
the C1-C4 axial hydrogens.
|
Problem set
Return to index